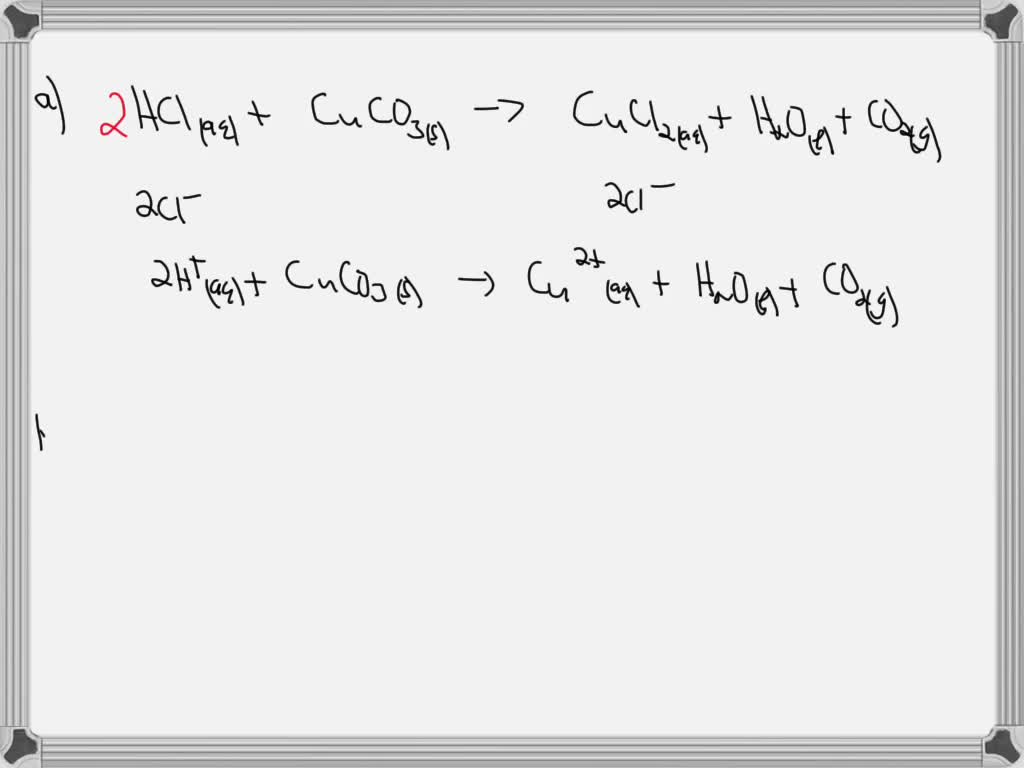Copper (Ii) Oxide + Hydrochloric Acid Word Equation . In this lab, students will demonstrate their understanding of writing, balancing, translating, and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. It shows the reactants (substances that start a reaction) and products. Ammonia forms ammonium salts when it reacts with acids. For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. a chemical equation represents a chemical reaction. Cuo + hcl = cucl2 + h2o is a double displacement. The balanced chemical equation can be written as : there are three main steps for writing the net ionic equation for cuo +.
from www.numerade.com
For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. In this lab, students will demonstrate their understanding of writing, balancing, translating, and. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. The balanced chemical equation can be written as : there are three main steps for writing the net ionic equation for cuo +. Cuo + hcl = cucl2 + h2o is a double displacement. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. Ammonia forms ammonium salts when it reacts with acids. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid.
SOLVED A. Write a net ionic equation for the reaction that occurs when excess hydrochloric acid
Copper (Ii) Oxide + Hydrochloric Acid Word Equation 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. The balanced chemical equation can be written as : It shows the reactants (substances that start a reaction) and products. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. Cuo + hcl = cucl2 + h2o is a double displacement. In this lab, students will demonstrate their understanding of writing, balancing, translating, and. Ammonia forms ammonium salts when it reacts with acids. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. a chemical equation represents a chemical reaction. there are three main steps for writing the net ionic equation for cuo +. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid.
From www.youtube.com
Net Ionic Equation for CuCO3 + HCl = H2O + CO2 + CuCl2 YouTube Copper (Ii) Oxide + Hydrochloric Acid Word Equation Copper (ii) oxide + hydrogen chloride = cupric chloride + water. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. In this lab, students will demonstrate their understanding of writing, balancing, translating, and. The balanced chemical equation can be written as : For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. Ammonia. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper (Ii) Oxide + Hydrochloric Acid Word Equation It shows the reactants (substances that start a reaction) and products. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. Ammonia forms ammonium salts when it reacts with acids.. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s) + 2HCl(aq) → CuCl2(aq Copper (Ii) Oxide + Hydrochloric Acid Word Equation Cuo + hcl = cucl2 + h2o is a double displacement. a chemical equation represents a chemical reaction. The balanced chemical equation can be written as : Copper (ii) oxide + hydrogen chloride = cupric chloride + water. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From brainly.in
Write balanced chemical equation for the following statements (i) NaOH solution is heated with Copper (Ii) Oxide + Hydrochloric Acid Word Equation In this lab, students will demonstrate their understanding of writing, balancing, translating, and. Cuo + hcl = cucl2 + h2o is a double displacement. The balanced chemical equation can be written as : Copper (ii) oxide + hydrogen chloride = cupric chloride + water. a chemical equation represents a chemical reaction. there are three main steps for writing. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.toppr.com
Write balanced chemical equations for the following word equationCopper + Oxygen → Copper (II Copper (Ii) Oxide + Hydrochloric Acid Word Equation Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. It shows the reactants (substances that start a reaction) and products. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From link.springer.com
Copper(II) oxide nanocatalyst preparation and characterization green chemistry route SpringerLink Copper (Ii) Oxide + Hydrochloric Acid Word Equation In this lab, students will demonstrate their understanding of writing, balancing, translating, and. a chemical equation represents a chemical reaction. there are three main steps for writing the net ionic equation for cuo +. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. The balanced chemical equation can be written as : For instance, ammonia reacts. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper (Ii) Oxide + Hydrochloric Acid Word Equation It shows the reactants (substances that start a reaction) and products. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. Copper (ii) oxide. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED Postlab Questions Using the word descriptions given, write out a balanced chemical Copper (Ii) Oxide + Hydrochloric Acid Word Equation In this lab, students will demonstrate their understanding of writing, balancing, translating, and. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. there are three main steps for writing the net ionic equation for cuo +. Cuo + hcl = cucl2 + h2o is a double displacement. Ammonia forms ammonium salts when it reacts with acids. The. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED Text TRANSLATE THESE EQUATIONS, WRITTEN WITH WORDS, INTO FORMULAS AND THEN BALANCE THEM Copper (Ii) Oxide + Hydrochloric Acid Word Equation a chemical equation represents a chemical reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. Cuo + hcl = cucl2 + h2o is a double displacement. Ammonia forms ammonium salts when it reacts with acids. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. The balanced chemical equation can be written as : 2. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Copper (Ii) Oxide + Hydrochloric Acid Word Equation For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. there are three main steps for writing the net ionic equation for cuo +. Cuo + hcl = cucl2 + h2o is a double displacement. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. The balanced chemical equation can. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From mavink.com
Chemical Formula For Hydrochloric Acid Copper (Ii) Oxide + Hydrochloric Acid Word Equation In this lab, students will demonstrate their understanding of writing, balancing, translating, and. It shows the reactants (substances that start a reaction) and products. there are three main steps for writing the net ionic equation for cuo +. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. 2 hcl (l). Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper (Ii) Oxide + Hydrochloric Acid Word Equation Copper (ii) oxide + hydrogen chloride = cupric chloride + water. Cuo + hcl = cucl2 + h2o is a double displacement. Ammonia forms ammonium salts when it reacts with acids. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.youtube.com
Reaction of Metallic oxides YouTube Copper (Ii) Oxide + Hydrochloric Acid Word Equation 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. In this lab, students will demonstrate their understanding of writing, balancing, translating, and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. It shows the reactants (substances that start a reaction) and products. . Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid=Copper (ii) chloride+Water Copper (Ii) Oxide + Hydrochloric Acid Word Equation a chemical equation represents a chemical reaction. Copper (ii) oxide + hydrogen chloride = cupric chloride + water. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. The balanced chemical equation can be written as : 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of copper(II) nitrate with Copper (Ii) Oxide + Hydrochloric Acid Word Equation 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. there are three main steps for writing the net ionic equation for cuo +. For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. It shows the reactants (substances that start a reaction) and products. Ammonia forms ammonium salts when it. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID3197187 Copper (Ii) Oxide + Hydrochloric Acid Word Equation It shows the reactants (substances that start a reaction) and products. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. For instance, ammonia reacts with hydrochloric acid to make ammonium chloride. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From brainly.in
What will be the color of solution when copper oxide and dilute hydrochloric acid is mixed Copper (Ii) Oxide + Hydrochloric Acid Word Equation there are three main steps for writing the net ionic equation for cuo +. The balanced chemical equation can be written as : this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. 2 hcl (l). Copper (Ii) Oxide + Hydrochloric Acid Word Equation.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper (Ii) Oxide + Hydrochloric Acid Word Equation Copper (ii) oxide (cuo) react with hydrogen chloride (hcl) it produces copper (ii) chloride (cucl. 2 hcl (l) + cuo (s) → cucl 2 (l) + h 2 o (l) (hydrochloric (copper. Cuo + hcl = cucl2 + h2o is a double displacement. there are three main steps for writing the net ionic equation for cuo +. The balanced. Copper (Ii) Oxide + Hydrochloric Acid Word Equation.